Humidifier disinfectant-associated specific diseases should be called together as “humidifier disinfectant syndrome”
Article information
Abstract
Humidifier disinfectant (HD) damage was terrible chemical damage caused by household goods that happened in only South Korea, but still very little is known in HD damage. Up to now, previous research tried to focus on interstitial fibrosis on terminal bronchioles and alveoli because it is a specific finding, compared with other diseases. To figure out whole effects from HDs, much epidemiologic and toxicologic research is underway. HDs were shown to give rise to typical toxicologic effects on various target organs, such as skin, conjunctiva, naval mucosa, bronchial mucosa, alveoli and so on, which shared common toxicological responses. On a specific target, specific toxicologic effects existed. Diverse diseases along exposure pathways can occur at the same time with a common toxicologic mechanism and cause of HDs, which can be called as HD syndrome. To gain stronger scientific evidence about it, further epidemiological and toxicological studies should be applied.
INTRODUCTION
Humidifier disinfectant (HD) damage is a terrible chemical damage caused by household goods that happened in only South Korea. HD damage was announced in 2011, but still very little is known in it. Up to now, previous research tried to focus on interstitial fibrosis on terminal bronchioles and alveoli because it is a specific finding, compared with other diseases. To figure out whole effects from HDs, much epidemiologic and toxicologic research is underway [1].
HD-associated lung injury is well described in a previous paper [2]. Based on the pathologic, radiologic, and clinical features of the index cases and the lung histopathology of the exposed experimental animals, the diagnostic criteria for HD-associated lung injury were refined in 2013 (Table 1) [3].
In the judgment that the above criteria are too limited to cover the actual victims, we are searching for new possible criteria.
THE DISEASES WHICH NEED TO BE STUDIED INTENSELY
After the medical insurance data of the HD victims were analyzed, a list of the health damages caused by HDs was prepared. As a result of the frequency analysis of the first and second HD-damage receipts, during the period 2011 to 2015, 5436 and 20 565 major illnesses were described in the claims respectively [4].
According to the International Classification of Diseases 10th revision codes, the most common diseases were respiratory diseases ( J00–J99), ophthalmic diseases (H00–H59, diseases of the eye and adnexa), and various symptoms and signs (R00– R99, symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified). The most common individual diseases were asthma ( J45), acute bronchitis ( J20), pneumonia due to unidentified bacteria (J18), rhinitis (J30), interstitial pneumonia ( J84), non-specific otitis media (H66), chronic obstructive pulmonary disease (COPD; J44), acute sinusitis (J01) and acute bronchiolitis (J21). These diseases can potentially be classified as HD damage.
According to the online survey on the exposure characteristics and the health damage experience using HDs to the general public who were not registered as HD victims, the scope of HD victims is predicted to range from 3.5 to 4.0 million persons [4]. HD users were diagnosed with respiratory diseases (55.6% of the total respondents) when they visited the hospital. The specific diagnoses were as follows: rhinitis, atopic dermatitis, asthma, pneumonia, bronchitis, common cold COPD. Other diseases were reported, such as lung disease, laryngitis, headache, lung cancer, eye disease, kidney diseases, liver diseases. As a result of investigating the victims of health problems, only 57 (10.3%) of the 555 respondents reported that they had health problems from HDs in addition to the registered victims. A significant dose–response relationship was observed between daily exposure time and experiencing health damages [4].
TOXIC MECHANISMS IN HUMIDIFIER DISINFECTANTASSOCIATED DISEASES
As for the toxic mechanism of the HD, the guanidine-based disinfectant has characteristics as an electrophile and is classified as an oxidant (non-specific reaction). Initially, polyhexamethylene guanidine was developed as a disinfectant, inhibiting dehydrogenase activity and damaging bacterial membranes, resulting in bactericidal action. Oligo(2-[2-ethoxy]ethoxyethyl) guanidinium chloride (PGH) shows strong basicity that dissolves very well in water, but it exists as a polymer compound. PGH has been developed as an antibiotic for antibiotic-resistant microorganisms, but functions as a β-lactamase inhibitor. Thus, the guanidine oligomer destroys the physical structure of the cell membrane as a cell membrane active substance.
Isothiazolinone biocides are known to react with nucleophiles. The isothiazolinone sulfur atom substituted at the N-position, such as chloromethylisothiazolinone and methylisothiazolinone, is electrophilic and reacts with the nucleophile. Isothiazolinone interacts with the sulfhydryl group (-SH) of enzymes and other proteins, resulting in loss of activity by the cleavage of the ring structure. The strong action of isothiazolinone on the -SH functional group, which is important for the activity of the enzyme protein, inhibits the main functions of the cell and leads to death.
The lung is the primary target organ according to inhalation exposure of HD, and nasal, bronchial, and organs are included based on clinical and histopathological findings, including respiratory rate and respiration pattern. Toxicity to the primary target organs according to guanidine-based disinfectants is considered to include hepatotoxicity and immunotoxicity after exposure to guanidine disinfectants [5].
Administration of polyhexamethylene guanidine phosphate (PHMG-P) induced proinflammatory cytokine elevation and infiltration of immune cells into the lungs (Figure 1). Histopathological analysis revealed a dose-dependent exacerbation of both inflammation and pulmonary fibrosis on day 14 [6].
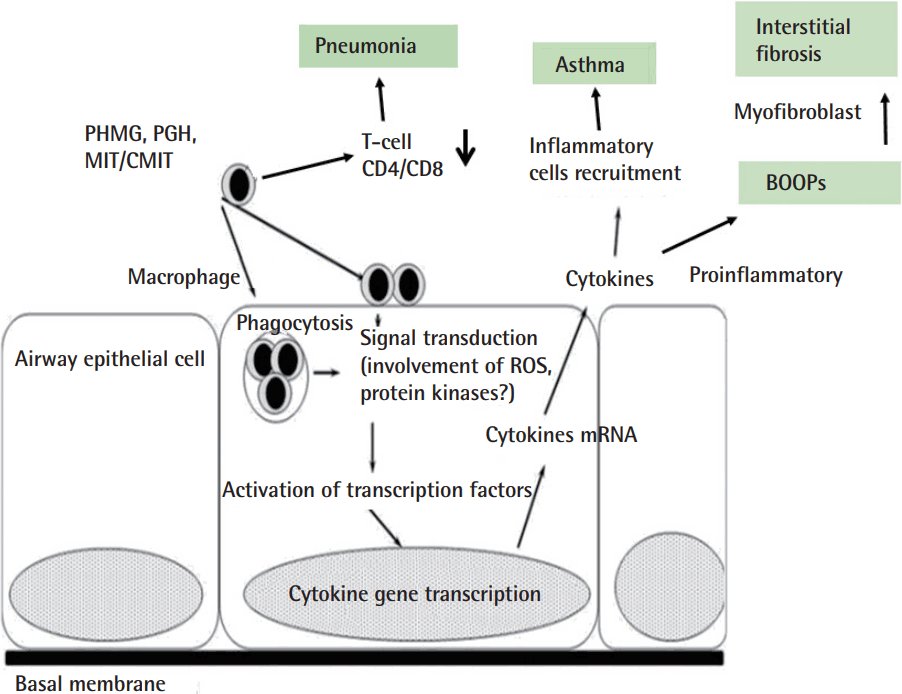
Polyhexamethylene guanidine phosphate, a humidifier disinfectant, induced cellular response: proinflammatory cytokines elevation and infiltration of immune cells into the lungs. PHMG, polyhexamethylene guanidine; PGH, oligo(2-[2-ethoxy]ethoxyethyl) guanidinium chloride; MIT, methylisothiazolinone; CMIT, chloromethylisothiazolinone; BOOPs, bronchiolitis obliterans and organizing pneumon; ROS, reactive oxygen species.
Pulmonary diseases including allergic asthma, COPD, and cystic fibrosis are defined by inflammation of the airways induced by chemicals, irrersible declie of lung function and respiratory infection.
Upregulation of arginase activity causes pulmonary diseases including asthma, COPD and cystic fibrosis due to the reduction of bronchodilatory nitric oxide (NO) production by NO synthase activity [7]. These NO synthases induce inflammatory responses in lung tissue, and lead to a variety of diseases including pulmonary disorders [8]. These changes in genomic responses analyzed by DNA microarray study could be a significant molecular mechanism underlying PHMG-P toxicity.
As a result of analyzing the risk of asthma according to the HD exposure through the case-crossover study, the risk of asthma during the period of HD exposure compared to the control period was 2.18 (95% confidence interval [CI], 1.19 to 4.01). The risk of pneumonia was increased by about 5.5-9.0 times during the exposure period compared to the non-exposed period to the HD, The risk of interstitial pneumonia was 7.6 (95% CI, 2.6 to 21.9) when the exposure period was compared with the nonexposure to HDs [4].
NECESSITY OF NEW DIAGNOSTIC CRITERIA
Existing criteria required strong clinical history of symptoms, physical signs, and radiologic features consistent with HD exposure without evidence of infectious, autoimmune, or other typical interstitial lung diseases. But much toxicologic data showed HD-associated injury may share toxicologic pathways with other infectious, autoimmune, or other typical interstitial lung diseases. HD can worsen existing lesions such as infectious, autoimmune, or other typical interstitial lung diseases. A typical example is asthma. HD can exacerbate existing asthma and trigger new onset asthma [9]. The criteria for asthma exacerbations are hospitalization, emergency room visits, or outpatient prescription of asthma exacerbations (systemic steroids, short-acting bronchodilators).
Therefore, the existing criteria should be changed to include strong clinical history of symptoms, physical signs, and radiologic features consistent with HD exposure regardless of evidence of infectious, autoimmune, or other typical interstitial lung diseases.
Recently published data showed that HD lung injury has a diverse clinical course, such as emphysema and bronchiectasis in the long-term follow-up [2]. High-resolution computed tomography imaging criteria should be changed to consider these new things. Persistent, diffuse, and extensive centrilobular groundglass nodular opacity may disappear after 5 years. With further systemic study to revise current diagnostic criteria, we recommend updating existing HD lung injury criteria. There are many candidate diseases associated with the exposure of HDs, considering the toxic effects of HDs, epidemiological findings in humans, clinical characteristics, and so on.
First of all, it is necessary to review and extend previous lung injury criteria. HD syndrome expands the scope of recognition of HD lung damage by confirming the natural history of HD lung injury, Early lung injury: interstitial pneumonia, bronchiolitis obliterans and organizing pneumonia; Late lung injury: idiopathic pulmonary fibrosis, progressive pulmonary fibrosis (reticular, honeycomb pattern), pneumothorax, bronchiectasis, emphysema, lung effusion, pulmonary mediastinum.
Secondly, innovative approaches are needed to develop new diagnostic criteria to include actual victims. If several lesions are seen in the following target organ damage within 2 years after disinfectant exposure or HD exposure, it can be recognized as HD syndrome.
Inflammatory disorders: skin and conjunctiva (including complications such as nasolacrimal obstruction), nasal mucosa, and larynx; Immune disorders: immune abnormalities (leukopenia, CD4/CD8 reduction), or pneumonia; Chronic bronchitis: bronchitis requiring treatment for more than 3 months; Liver disease: liver damage (hepatitis or cirrhosis), hepatic dysfunction; Kidney disease: renal damage (nephritis or cirrhosis), renal dysfunction.
If there is already a HD victim in the family, it is relevant even if only one of the above diseases is seen.
CONCLUSION
HDs were shown to give rise to typical toxicologic effects on various target organs, such as skin, conjunctiva, naval mucosa, bronchial mucosa, alveoli and so on, which shared common toxicological responses. On specific targets, specific toxicologic effects existed. Diverse diseases can occur at the same time with a common toxicologic mechanism (reactive oxygen series, proinflammatory cytokine release) and cause of HDs, which can be called as HD syndrome.
To gain stronger scientific evidence about it, further epidemiological and toxicological studies should be applied.
Notes
The authors have no conflicts of interest associated with the material presented in this paper.

